Montana Biotech Companies to Watch 2020
By Christina Henderson, Samuel Boudreau, and Martina Pansze
Amid the economic turbulence of 2020, Montana’s biotechnology industry has risen to the unique challenges presented by COVID-19.
Many companies have adjusted their labs and office routines to safely continue their work on cancer research, pain management solutions, or development of cell culture media. Others have capitalized on the opportunity to create technological solutions to the pandemic’s obstacles, such as Missoula’s Ahana, which brings telehealth options to rural Montana communities.
Many Montanan ventures are working with the virus directly at the forefront of research and testing improvement efforts. Missoula’s Rocky Mountain Biologicals created Viral Transport Medium for coronavirus testing kits. Bozeman’s Golden Helix developed bioinformatics software to automate the diagnostic process. Missoula-based PatientOne is working with the county health department to implement software that automatically updates patient symptoms and temperature information to the government database. Also in Missoula, FYR Diagnostics has developed a COVID sample processing system that cuts standard testing times by half.
Since 2017, the Montana High Tech Business Alliance has released annual Montana Companies to Watch lists. These articles have highlighted the state’s fastest-growing and most innovative tech businesses, including 2019 Montana Startups to Watch and 2019 Montana High-Growth Companies to Watch. 2020 is the first year that the Alliance is publishing a Montana Biotech Companies to Watch list in partnership with the Montana Bioscience Cluster Initiative.
The caliber of companies selected as 2020 Montana Biotech Companies to Watch reflects the steady long-term growth of the state’s bioscience ecosystem. This phenomenon was recognized nationally at the end of 2019 when the U.S. Small Business Administration made one of seven national awards to the Montana Bioscience Cluster Initiative.
The 2020 Montana Biotech Companies to Watch reveal a number of key industry trends:
Four of the 11 firms have raised venture capital investment, a sign of growing interest in Montana bioscience from investors like Michael Goguen, founder of Two Bear Capital in Whitefish.
Four companies were awarded SBIR/STTR grants, reflecting Montana’s #1 position in securing NIH awards. In 2019, Montana’s SBIR/STIR Application Success Rate with NIH was 48.3 percent - more than double the national average - with support from organizations like TechLink in Bozeman.
The role of universities in fueling biotech growth is evident in the locations of company headquarters. Four firms are in Bozeman near MSU and four are in Missoula close to UM. Two firms call the Flathead Valley home, indicating the rise of that region as an emerging hub for biotech.
Montana’s biotech firms are innovators in a range of fields vital to the life sciences - vaccine development, diagnostic testing, bioinformatics, telemedicine, medical devices, cancer treatment, pain management, and development of cell culture media.
More than half of selected firms are rising up to meet new needs in healthcare driven by the coronavirus pandemic.
Here are our picks in alphabetical order:
NanoValent Pharmaceuticals, Bozeman
Rocky Mountain Biologicals, Missoula
VIRIS Detection Systems, Bozeman
We received nominations from industry partners across Montana, including MonTEC, Montana Bioscience Alliance, Missoula Economic Partnership, University of Montana, Montana State University, Kalispell Chamber of Commerce, Two Bear Capital, Next Frontier Capital, Goodworks Ventures, Blackstone LaunchPad, and Montana World Trade Center. Our 11 finalists were selected based on the following criteria:
Steep revenue growth and/or are working in a high-growth sector
Poised to launch high-potential products or services
Own or are developing valuable intellectual property
On track to land major clients or enter new markets
Plan to expand operations or add a significant number of jobs in the next year
Have management teams led by experienced entrepreneurs or top experts in their fields
Working to address unique challenges presented by COVID-19
Ahana
Although only a year old, Ahana is providing pediatric care for many Montana communities. This software and video-based platform allows patients to receive care during non-traditional hours (like weekends and evenings), assists healthcare facilities in keeping records of medically-complex and underserved cases, and has a photo upload feature to keep doctors updated with current conditions. Photo via Ahana.
Location: Missoula, MT
What they do: A collaboration of Montana pediatricians bringing video visits to children and parents during non-standard business hours.
Why we’re watching them: As a pediatrician, Chelsea Bodnar saw a problem for many small, rural communities in Montana: families don’t have access to effective, efficient, and convenient pediatric care.
In 2019, Bodnar launched Ahana Pediatrics, a software that facilitates video appointments for local and rural patients during evenings and weekends. Ahana’s platform allows healthcare providers to keep detailed records of every patient, including traditionally underserved, medically-complex cases.
Ahana offers a number of additional benefits to healthcare providers and patients unavailable through the traditional model of in-office care. For example, parents can contact a doctor on the weekend to address concerns about a child's post-op recovery without having to make a trip to the ER. And physicians can evaluate a patient's condition remotely through live video, or in photos uploaded to the Ahana platform.
Due to COVID’s socially-distant restrictions, Ahana Pediatrics has seen an uptick in use because of its virtual application. A parent company of Blue Cross Blue Shield of Montana has recently invested in Ahana’s work. Ahana has been approached by multiple states and healthcare organizations to implement their software, but are currently only focusing on expanding in Montana communities.
A fifth-generation Montanan, Bodnar attended Harvard Medical School and The University of Oxford as a Rhodes Scholar. After Residency at Seattle Children's Hospital/University of Washington, she was also a health policy fellow at the Institute of Medicine, and Director of Children's Healthcare quality at a large consortium of Federally Qualified Health Centers. She returned home to Missoula in 2018 when her husband, Seth Bodnar, became president of the University of Montana.
With financial support from a Robert Wood Johnson Foundation grant, Bodnar joined forces with pediatricians from across the state to use Ahana's platform to create access to pediatric-specific care in Fort Peck, a rural community of less than 300 people in eastern Montana. The predominant frontline health facility in Fort Peck is based in the middle school health center, which has unused, top-notch medical equipment. Ahana integrated their software into this equipment to enable pediatric care and adequate screenings for all patients.
Ahana is also planning on expanding their infrastructure beyond pediatrics to include primary care patients. Ahana, Inc. currently employs 4 people and plans to grow.
Where are they now? - Inimmune
Location: Missoula
What they Do: Discover and develop new therapeutics for allergy, autoimmunity, infectious disease, and cancer
We covered Missoula-based biotech therapeutics company Inimmune in our 2018 Startups to Watch List. In the past two years, Inimmune has grown rapidly and transitioned from a pre-clinical to clinical stage company set to advance four lead programs to human clinical trials in the next 3-4 years. Since 2018, they have also:
Secured $22 M Series A investment from Two Bear Capital to advance multiple lead programs through Phase I human clinical testing.
Awarded over $30 M in new NIH research grants and contracts through collaborative vaccine research partnerships.
Expanded and accelerated their drug discovery pipeline with lead programs in immuno-oncology, allergy, opioid addiction vaccines and infectious disease vaccines.
Expanded their research and development efforts in Missoula with key new hires across all research areas.
Received 4 Small Business Innovation Research grants from NIH totaling $3.7M in research funding.
FYR Diagnostics
CEO Chris Booth (left) and President Sarj Patel (right) discussing their simplified COVID-19 test with Governor Steve Bullock (center) this past April. FYR’s test processing capabilities were expanded in October 2020. Photo via Tom Bauer for The Missoulian.
Location: Missoula, MT
What they do: Develop novel diagnostic tools for human and agricultural diseases.
Why we’re watching them: FYR Diagnostics is at the forefront of COVID-19 testing in Montana. In October, the company announced that it would ramp up large-scale COVID-19 test processing in its new high-capacity lab in Missoula to help relieve testing bottlenecks in the state.
FYR provides sample collection kits to businesses, universities, and healthcare providers who then send the samples back for test processing. The team is also working on a simplified COVID test, which takes only 30 minutes to run as opposed to the standard 1.5-2+ hours.
Founded in 2016, FYR Diagnostics began as a laboratory developing a portfolio of early-detection tests for cancers, neurological disorders, and other human diseases. In 2018, they received a more than $300,000 SBIR grant from the National Institutes of Health to create an advanced epilepsy diagnostic test.
The fast-growing startup has also been supported by funding from Two Bear Capital in Whitefish as well as other grants. FYR has licensed biomarker-detecting technology in collaboration with Montana State University and diversified into applications for crop and livestock diseases in agriculture.
FYR’s leadership team boasts extensive experience in life sciences research and entrepreneurship. CEO Chris Booth holds a PhD in Biochemistry, Cellular, and Molecular Biology from Johns Hopkins School of Medicine. President Sarj Patel earned his PhD in Neuropharmacology from the University of Montana. Senior Research Scientist Vik Ghai earned his PhD in Molecular Biology from the University of Calgary. And COO David Booth is a serial entrepreneur who founded several previous companies, including Expesicor, a Montana biotech company developing therapies and imaging agents for neurological disorders.
FYR anticipates rapid growth of the 15-person team over the next year as FYR continues to address COVID and implement other detection tests. FYR is developing a training program for certified clinical laboratory technicians to help meet the demand. They also plan to implement an agricultural grant beginning January 2021.
Golden Helix
Golden Helix’s Cytoban Plot program displays a view of the bands within a chromosome. The bioinformatics software company serves over 20,000 users, including researchers at Johnson & Johnson, UCLA, Children’s Hospital of Los Angeles, Rutgers, and the National Eye Institute. Photo via Golden Helix.
Location: Bozeman, MT
What they do: A bioinformatics company specializing in genomic data analysis.
Why we’re watching them: Since 2017, Golden Helix has seen 110% growth. The Bozeman-based venture was listed on Inc. 5000’s List of Fastest-Growing Companies for the second consecutive year this August and secured the Global Health and Pharma Award during the 2020 Biotechnology Awards.
Golden Helix’s software offers integrated and automated data reporting used to diagnose and research diseases by matching them to a patient’s genetic makeup.
The software platforms, which have been cited in over 1,300 peer-reviewed publications, are used for pharmaceutical research, agricultural genomics, education, human genetic research, and laboratory testing.
Golden Helix programs can be used to automate the bioinformatics process for COVID-19 and more rapidly turnaround diagnosis data. Golden Helix was recently published in the Journal of Precision Medicine for their work diagnosing and tracking coronavirus infections through next-generation gene sequencing.
In 2018 and 2019, Golden Helix was awarded two SBIR grants totaling over $3.3 million from the National Institutes of Health to investigate Next-Generation Sequencing (NGS) for clinical testing and diagnostics. NGS is an umbrella term for modern sequencing technologies that collect unique virus data. Using NGS, a process called dendrogram analysis can determine infection chains useful in identifying virus outbreaks. Used in tandem with old-fashioned contact tracing, NGS offers a complete overview of the virus’ infection process and more detail in studying mutations and isolates’ evolutionary lineage.
The global firm has grown to serve over 20,000 users. Golden Helix customers include Johnson & Johnson, Lilly, University of Illinois, University of Iowa, University of New Mexico, UCLA, Children’s Hospital of Los Angeles, Rutgers, and the National Eye Institute.
Golden Helix’s most successful products include VarSeq, which was developed to analyze data sets for tertiary analysis, and SNP & Variation Suite—an analytic tool that allows researchers to perform complex analyses and visualizations on genomic and phenotypic data. Sherer and his team are also working to embed AI capabilities into their platforms.
In addition to expanding their work with clinicians focused on COVID-19, the company plans to accelerate its engagement with international customers in Europe, Asia, Australia, the Middle East, and South America.
NanoValent Pharmaceuticals
An illustrated example of the 70nm wide nanoparticles used in NanoValent treatments. CEO Timothy Enns lovingly refers to the antibody-clad fat bubbles as “death stars for cancer.” Photo via PBS.
Location: Bozeman, MT
What they do: Research and develop targeted nanosphere therapies for cancer treatments
Why we’re watching them: Co-founded by a board-certified pathologist with expertise in childhood tumors and a PhD organic chemist with 7 patents to his name, NanoValent is developing new therapies for hard-to-fight cancers.
Timothy Triche MD, PhD, co-director of the Center for Personalized Medicine at the Children's Hospital of Los Angeles, partnered with Jon Nagy, PhD, a veteran of Bozeman-based vaccine-maker Ligocyte Pharmaceuticals, to launch NanoValent in 2006. Dr. Triche was also a principal and founder in several previous ventures that have raised a total of $70M.
NanoValent Pharmaceuticals’ Bozeman lab is advancing a novel generation of nanoparticle-based drugs called targeted nanosphere (TNS) products. Nanoparticles are microscopic fat bubbles targeted with antibodies to attack cancer without the drug affecting the patient’s healthy, non-cancerous cells.
NanoValent has raised over $5 million, including two National Institutes of Health SBIR grants awarded in 2018 totaling $4 million. The first grant, carried out primarily with the Children’s Hospital of Los Angeles, supports development of a safer and more effective therapeutic for adolescents with Ewing sarcoma. The second grant, in collaboration with Boston University, applies NanoValent’s core technology to non-oncology surgical adhesions.
Additional funding has come from direct management investment, angel investors and grants from the National Science Foundation, the National Cancer Institute, and the Montana Chamber of Commerce.
Much of NanoValent’s TNS technology is protected by intellectual property law. Most recently, in January of 2020 NanoValent announced the publication of US Patent Targeted Polymerized Nanoparticles for cancer treatment.
NanoValent’s team, which includes three employees at their Bozeman lab, collaborates with healthcare and academic partners to develop therapies using their technology. Funded mostly through grant work, NanoValent advances their products to the point of commercial feasibility and then works with contract research organizations or partner labs to move products out of the scientific advancement phase and into the commercial realm.
NanoValent has created a proprietary platform that can also target other cancers and medical conditions.The company creates a particularly useful nanoparticle known as Hybrid Polymerized Liposomal Nanoparticles (HPLN) that enhances existing cancer therapies. HPLN targeted nanospheres work well for antibody, immunotherapy, and antibody-drug conjugate treatments. NanoValent has so far developed three HPLN-based TNS candidates with the Children’s Hospital of LA that are focused primarily for use in pediatric oncology.
CEO Timothy Enns, who joined the company in 2016, offers experience with entrepreneurship and sales from his 34 years in the pharmaceutical industry and related business development positions. Although much of the company’s leadership, including Triche and Enns, work virtually from out-of-state, NanoValent maintains its headquarters in Bozeman.
NanoValent’s leading drug candidate, NV103 for treating Ewing Sarcoma, is approaching Phase I clinical trials in 2021.
PatientOne
PatientOne Co-Founder John O’Connor, CEO Jeff Fee, and CTO Erik Guzik (left to right) after securing a $1.2M Seed Round led by Dundee Venture Capital and Revolution’s Rise of the Rest Seed Fund in 2019. Although initially known for their videos preparing patients for operations, PatientOne is expanding into the Remote Patient Monitoring (RPM) software sector. Photo via The Missoulian.
Location: Missoula, MT
What they do? Develop virtual tools and operational remote monitoring technology for primary care.
Why we’re watching them: If you’ve had surgery in or around Missoula recently, you probably watched a video outlining how to prepare and recover from your operation. This training was brought to you by PatientOne, a company that specializes in technology for operative care. The software start-up is now branching out into the telemedicine and Remote Patient Monitoring (RPM) worlds and will be implementing RPM software across hospitals and healthcare facilities across the country in a matter of weeks.
PatientOne has partnered with the Missoula County Health Department (MCHD) to combat COVID-19. The company is developing software that will automatically update MCHD with patient symptoms and temperatures, thereby reducing nurse and healthcare worker exposure to the virus. As hospitals face resource constraints, Patient One hopes this public/private collaboration will decrease the pressure on Montana’s healthcare system.
In 2019, PatientOne raised a $1.2M seed round led by Dundee Venture Capital and Revolution’s Rise of the Rest Seed Fund, which was co-founded by former America Online (AOL) CEO Steve Case. Next Frontier Capital of Bozeman and Goodworks Ventures of Missoula also invested.
PatientOne completed the competitive BoomTown HealthTech Accelerator program in Boulder, Colorado in 2018 and was a part of the inaugural 2018 cohort of Blackfoot’s C2M Beta technology accelerator. PatientOne was also awarded $106,731 from the Big Sky Economic Development Trust Fund in May of 2019 to support the creation of 9 local jobs.
CEO and co-founder Jeff Fee was inspired to create PatientOne in 2018 after noticing inefficiencies during his 25 years of experience in the healthcare industry. Fee served a decade as chief executive for Providence Health and Services, Western Montana region. PatientOne’s co-founder and CTO, Erik Guzick, has a background developing virtual learning software for veteran PTSD research. Guzick is also the founder and CEO of VAST: Next Generation Learning, a SaaS online learning platform.
In a webinar hosted by the MHTBA in April, Fee said, “The COVID pandemic and crisis created almost an overnight understanding of the clinical and public health benefits of remotely monitoring at-risk patients in their home. My prediction is, I don't think that genie’s going back in the bottle.”
PurCell Bio
PurCell founder Dr. Elizabeth Corbin entered the stem cell research world when her son was diagnosed with a rare genomic disorder. On the way, she developed an alternative to fetal bovine serum supplements. Photo via Elizabeth Corbin.
Location: Bozeman, MT
What they do: Create supplements free from animal products that can replace blood products in cell culture.
Why we’re watching them: Fetal bovine serum (FBS), which is extracted from the blood of cow fetuses, is the main additive used in the majority of cell cultures.
Fetal bovine serum introduces at least nine different types of toxins and contaminants to cell lines, so products supplemented using FBS undergo a battery of sterility tests. PurCell’s cultures largely sidestep that costly process by eliminating the variability and contamination issues of animal serum.
CEO Dr. Elizabeth “Tess” D Corbin founded PurCell Bio in 2018 as she finished up her PhD in Biochemistry at Montana State University.
After years as a musician and entrepreneur, Corbin returned to her hometown of Bozeman as a divorced mother to finish a degree in finance at MSU. However, her son was diagnosed with a congenital enzyme disorder in 2006. Following the prognosis, Corbin began researching advanced metabolics and biochemistry to try to develop a cure for the rare condition and others like it. She eventually attended graduate school where she researched how to improve stem cell efficiency to support therapies for genetic disorders. As a doctoral student, Corbin won a Ford Foundation Dissertation Fellowship for her research supplementing cell culture media with fatty acids.
PurCell Bio’s methodologies eliminate fetal bovine serum from their cell culture by replacing it with precise mixes of fats and other nutrients tailored to different cell types. The challenge in developing alternatives to animal-derived serum is to ensure solutions support cell growth and proliferation. Initial tests of PurCell Bio’s research prototypes show equivalent or better proliferation than serum and available serum substitutes.
In May, PurCell Bio was the first place winner of the “traditional” category in the annual Montana State University $50k Venture Competition hosted by the Jake Jabs College of Business and Entrepreneurship, netting $15,000. The company has also secured funding through the ASCEND regional pitch event sponsored by Vertici of Seattle, the MSU CATalyst Gap Fund program, ITREP College of Medicine at the University of Vermont, and MSU’s TechLink office, and the Montana Bioscience Cluster Initiative’s 4th F Fund grant program. SiteOne Therapeutics has also contributed substantial in-kind support.
Formerly known as OptimaLabs, PurCell’s cultures have myriad uses downstream in the market including advanced human therapies, vaccine development and "lab meat". The venture hopes to begin commercializing their product line of four different nutrient mixes in January. In preparation for launch, the company is currently fine-tuning their manufacturing process, including implementing partial automation of complexation and ensuring compliance with national standards.
PurCell Bio currently employs 4 people, including two MSU students, and Corbin has plans to hire more Montanans as the company expands. PurCell Bio has huge potential for growth in a $1.3 billion annual industry for sera and other reagents.
Rocky Mountain Biologicals
The sterile fill room in the RMBIO facility is used to bottle products without introducing contaminates. The company’s cell culture media eventually become components of vaccines, clinical diagnostics, and cosmetics. Photo via RMBIO.
Location: Missoula, MT
What they do: Manufacture cell culture media and protein fractions for pharmaceutical and biotechnology industries
Why we’re watching them: Since late March, Rocky Mountain Biologicals (RMBIO) has been producing medium used for COVID-19 testing kits. When patients are tested, the swab is placed in a tube with a small amount of liquid customized to preserve the viral RNA while the sample is transferred to a testing facility. The liquid, called Viral Transport Medium (VTM), is manufactured under CDC protocol and tested at RMBIO for final sterility. RMBIO is conducting internal validation studies that aim to extend the amount of time that the VTM can be stored without refrigeration—making the medium more widely usable at non-lab facilities such as drive-up testing sites.
VTM production has allowed RMBIO to tap into the medical supply industry and create partnerships with distributors that cater directly to hospitals and clinics. In doing so, the company has broadened their customer base exponentially and created new outlets for their standard line of products.
RMBIO’s lab creates cell culture media components and animal protein fractions. Much of the Missoula-based company’s products are used in cell culture and stem cell research, living up to the company’s tagline: “our science makes your science better.” Protein fractions and serum products are also sold to downstream manufacturers to become components of vaccines, clinical diagnostics, veterinary care, and even cosmetics. RMBIO’s clients include Google, Stanford University, National Institutes of Health, 3M, and Pfizer.
The Missoula facility has 13 full-time employees, including production, quality assurance, quality control, sales, and customer service departments. COO Jeremy Amberson describes the small team as nimble and able to respond to market needs quickly. The team is currently developing a protein-free media line of products that don't rely on extensive processing.
Founded in 2004 by Suresh Daniel and V.K. Daniel, pioneers of the serum and supplement fields, RMBIO was purchased in 2019 by a South Korean group seeking to gain a foothold in the protein side of biopharma. The acquisition created an opportunity for RMBIO to develop a larger market in Asia.
International investment has not changed RMBIO’s commitment to the growth of biotech in Western Montana. RMBIO helped found the Missoula Vaccine Partnership, an initiative that leverages local assets like research labs, universities, and thriving startups to establish the region as a hub for vaccine production.
“A key tenant of RMBIO is that Missoula has what it takes to house a biotech sector that is successful and competitive on a global scale,” said Amberson.
SOLO-DEX
An animated representation of SOLO-DEX’s Fascile® Catheter System. The catheter takes 5 minutes to implement and works for 72 hours post-procedure, eliminating the need for opioid pain management in most procedures. Photo courtesy of Steve Eror.
Location: Wolf Creek, MT
What they do: Designed an opioid-free catheter device for post-surgical pain relief
Why we’re watching them: Founded in Wolf Creek, Montana by two anesthesiologists, SOLO-DEX’s primary product is a patented Fascile® Catheter System that simplifies and accelerates continuous Peripheral Nerve Block (cPNB) placement in a 5-minute procedure.
In a country struggling with widespread opioid use and misuse, SOLO-DEX allows patients to experience comfort for up to 72 hours after their medical procedure without opioid use. After the 72 hour period, most patients using SOLO-DEX reported only needing over-the-counter pain relievers.
Ranging from $70-100, SOLO-DEX is more affordable than other pain-relieving options, which can cost more than $300. SOLO-DEX is FDA cleared, CE marked for sale in the European Union, and has been used to treat more than 7,000 patients to date.
The device was originally created by Dan Kopacz, MD and Sundar Rajendran, MD, who were working with Olympic athletes seeking quicker recovery times without steroid use. Kopacz was responsible for changing residency requirements in anesthesiology to include mandatory regional anesthesia training. Rajendran has had 20 years of practice focused on nerve block procedures for surgery. SOLO-DEX has unique features based on the founders’ doctoral experience, such as the ability for anesthesiologists to place a catheter with one hand as an ultrasound device is maneuvered in the other.
SOLO-DEX is clinically proven and validated by top healthcare and governmental organizations like Harvard Medical School, Stanford School of Medicine, Duke Medicine, The United States Army, and The United States Air Force.
CEO Steve Eror, who has had 26 years in the medical industry, hopes to begin widespread shipment from Wolf Creek to local and national healthcare facilities later this month.
Swan Valley Medical
Swan Valley Medical has been issued 32 U.S. and International patents with 4 patents pending on its T-SPeC® and related technology. Photo via Swan Valley Medical.
Location: Bigfork, MT
What they do: A mid-stage, privately held medical device company with a unique hospital solution using its patented T-SPeC® technology and advanced analytics to replace existing hospital-based Standards of Practice
Why we’re watching them: Swan Valley Medical was founded in Montana in 2006 by Ron Zook, a Montana native, MSU engineering graduate, MBA, and serial entrepreneur, and Dr. Kenneth High, a Montana urologist.
Zook was an aerospace design engineer before launching seven startups from concept, including a VC funded turn-around. Zook, also managed an international Japanese investment fund, focusing on M&A transactions and technology licenses.
Swan Valley Medical is engaged in changing a current Standard of Practice that was first initiated over 200 years ago with the invention of the flexible urethral catheter by Benjamin Franklin. Currently, over 17 million urethral Foley catheters are placed in U.S. hospitals annually. Catheter-Associated Urinary Tract Infections (CAUTI) are the single largest source of hospital-acquired infections worldwide, exceeding one million annually in the U.S. The company’s T-SPeC® technology enables the replacement of urethral catheters with suprapubic catheters in the targeted critical care patient, eliminating CAUTI infections, urethral catheter placement injuries, urosepsis and related mortality.
T-SPeC® represents a disruptive technology that could fundamentally transform how hospitalized patients are catheterized for bladder and fluids management worldwide. The annual market exceeds $15 billion in the U.S. and $50 billion internationally, with no competitors. The company projects over $700 million in annualized revenue with implementations in 230 U.S. hospitals.
Swan Valley Medical is using advanced analytics to develop customized informatics solutions. This enables individual hospitals and hospital networks to better understand the predicted clinical and economic benefits for the company’s proposed changes to existing Standards of Care.
The company has been issued 32 patents for its T-SPeC® and related technology and has received regulatory clearances from FDA, SFDA, CE Mark, Health Canada, Cofepris and ISO 13485. Market development has resulted with initial sales revenue in the U.S., E.U., Canada and Mexico. Final device manufacturing assembly and fulfillment is handled by its Denver location, with a U.S. based supplier network. The company is looking to transfer manufacturing to Montana.
The market value proposition results in shorter length of stay, reduced readmissions, prevention of mortality and ACA payment penalties, while improving patient satisfaction, saving individual hospitals millions of dollars annually.
Truwl
Funded by Two Bear Capital and the National Human Genome Institute, Truwl has built an online platform that allows researchers to share genomic data analysis methods. Photo via Truwl.
Location: Whitefish, MT
What they do: Develop bioinformatics software for sharing data analysis in genomic research.
Why we’re watching them: Technological advances in gene sequencing in the last decade have transformed the life sciences into a data-centric field. To gain insights from this new proliferation of data, however, biological researchers must navigate complex computational tools. Truwl is simplifying the process by building a platform that allows users to share their best methods for genomic data analysis with the wider scientific community.
In 2017, Truwl secured a seed round of funding from Michael Goguen, founder of Two Bear Capital in Whitefish.
In November 2019, the National Human Genome Research Institute, part of the National Institutes of Health Library (NIH), awarded Truwl $150k for the first phase of a Small Business Technology Transfer (STTR) grant. Truwl is collaborating with the ENCODE (ENCyclopedia Of DNA Elements) Data Coordinating Center (DCC) at Stanford University to demonstrate the ability to run advanced computational analyses through the online platform.
Truwl serves both the content producers inventing new ways to analyze genomic data and consumers who need to use them. Researchers from a variety of fields – agriculture, medicine, synthetic biology, conservation - can share bioinformatics content and get community feedback.
Truwl is search-engine-optimized, allows browsing without sign in, and tags content to link relevant information. The vision is to empower researchers at all levels to analyze their own data without having to become computer science experts.
Truwl president and co-founder Karl Sebby earned his PhD in Chemistry at Montana State University in Bozeman. Later, he worked as a postdoc at the National Institute of Standards and Technology in Boulder, CO and taught at Gonzaga University before moving to his wife’s hometown of Whitefish, Mont. Sebby met co-founder Tim Thompson, a software engineer, while working on a cancer research project in Whitefish. The pair launched Truwl (formerly xD Bio) in 2016 to address the barriers they faced trying to reproduce computational techniques in published research.
Thompson left Truwl in October 2020. In addition to Sebby, the team now includes two full-time software engineers and four contractors.
VIRIS Detection Systems
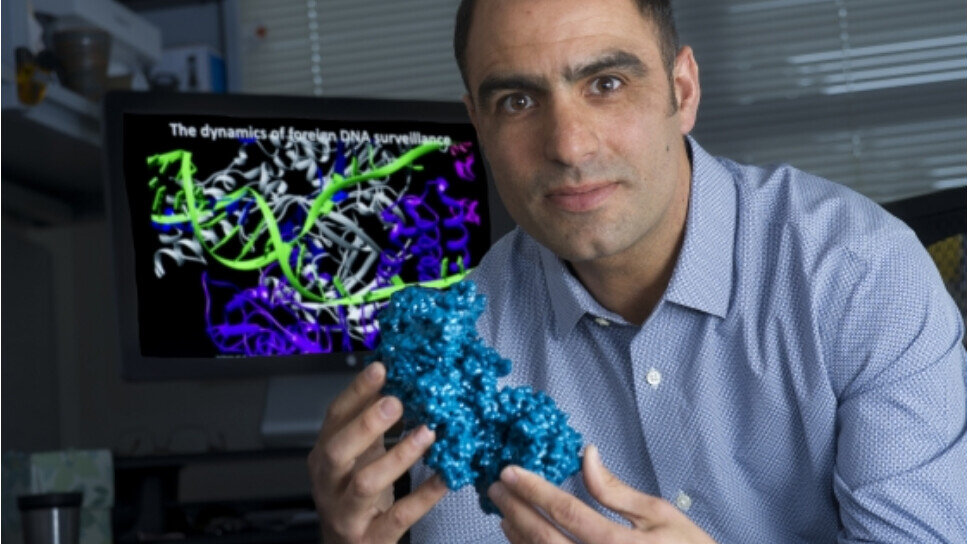
Dr. Blake Wiedenheft holding a CRISPR protein in his office in 2017. After working in the UC Berkeley lab of 2020 Nobel Prize winner Jennifer A. Doudna, Wiedenheft started his own research lab at MSU and launched VIRIS Detection Systems, a biotech startup using CRISPR to develop new detection tests for viruses like HIV, Ebola, and COVID-19. Photo taken by Kelly Gorham, MSU.
Location: Bozeman, MT
What they do: Develop viral diagnostics using CRISPR-based technologies.
Why we’re watching them: Since its discovery eight years ago, the precise gene-editing technology known as CRISPR has revolutionized the life sciences. CRISPR, which stands for Clustered Regularly Interspaced Short Palindromic Repeats, is used by researchers around the globe to create hardier plants and develop new treatments for diseases like sickle-cell anemia and cancer. In October 2020, Emmanuelle Charpentier and Jennifer A. Doudna shared the Nobel Prize in Chemistry for their discovery of CRISPR.
Dr. Blake Wiedenheft, associate professor in Microbiology and Immunology at Montana State University, has had a front row seat to this scientific breakthrough. Wiedenheft contributed to Dr. Doudna’s research as a postdoc in her lab at the University of California, Berkeley from 2007 to 2012. Wiedenheft’s doctoral research took him to Yellowstone National Park where he studied how bacteria fight off viruses in the boiling acid hot springs. Later, he returned to Montana to start his own CRISPR research program at MSU.
In April 2020, Wiedenheft launched VIRIS Detection Systems, a biotech startup using CRISPR to develop new diagnostic tools. VIRIS (Viral Identification Rapidly Issued Strips), Detection Systems is creating an adaptable detection test that is quick, accurate, and inexpensive. Near term goals are to develop a test for SARS-CoV-2 (the cause of COVID19), but the platform is versatile and can be quickly reprogramed for detection of other viruses (e.g., Ebola, Influenza, or HIV), genes associated with non-infectious diseases (e.g., oncogenes) or disease important to the agricultural industry.
In August 2020, VIRIS raised a seed funding round with Khosla Ventures, Next Frontier Capital, and MSU.
About the Publisher: Launched in 2014, the Montana High Tech Business Alliance is an nonpartisan nonprofit association of highly-engaged high tech and manufacturing companies and affiliates creating high-paying jobs in Montana. For more information, visit MTHighTech.org or subscribe to our biweekly newsletter.
About the Authors:
Samuel Boudreau is the Writer and Digital Content Coordinator Intern at the MHTBA. He graduated from Middlebury College with a degree in Gender, Sexuality and Feminist Studies and Psychology and is currently pursuing his MFA at The University of Montana while teaching Introductory and Intermediate Writing Courses.
Martina Pansze is the Communications Director for the Montana High Tech Business Alliance. She graduated from Whitman College with a degree in Film and Media Studies, and has worked as a freelance journalist and grant writer.
Christina Quick Henderson is founding executive director of the Montana High Tech Business Alliance and an instructor in the College of Business at the University of Montana.